When someone finally arrives where he is expected, he is told that "it was time." When parents have spent years paying for a vaccine that has been proven effective in preventing pneumonia, among other conditions, and finally decide to include it in the vaccination schedule, we say "It was already a year".
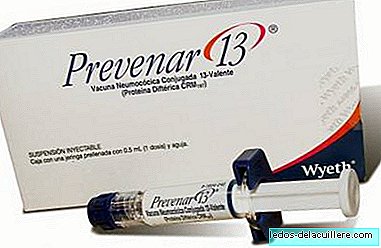
It was already a year. I already played. At last. Because it is a vaccine that prevents pneumococcus bacteria, which causes pneumonia, otitis and meningitis as common diseases and the Ministry of Health has decided Prevenar 13 becomes part of the state vaccination calendar as of 2016.
From 2016?
Of course, or did you think you lived in a serious country? Prevenar 13, before Prevenar 7, which for a time lived with Synflorix 10 (the number indicates the amount of serotypes included in the vaccine), is a vaccine that for a long time was part of the vaccine calendar of some autonomous communities.
Madrid is a clear example, and thanks to this mass vaccination all children were able to verify a reduction of bacteremic pneumonia of 72%, meningitis by 54% and empyema by 45%.
The curious thing is that while the Heracles study was being carried out, from which these figures are derived, the regional government of Madrid decided to stop subsidizing it.
In other autonomous communities the vaccine could only be given if the parents decided to put it, or if the parents who had decided to put it they had money to buy it. Those children whose parents did not have the nearly € 75 that the vaccine costs, multiplied by 4, of the four doses, were not immunized (nor are they currently being).
The vaccine only enters through social security in cases of babies with chronic diseases (heart disease, kidney disease, etc.). So yes, the vaccine is considered necessary. But for healthy children, well look, let the parents pay if they want, they should have thought.
Vaccination in 3 doses
Now, on the other hand, it seems that they have changed their mind and have decided that the vaccine will be part of the vaccine schedule within a year and a month.
The current administration schedule, when parents buy the vaccine, is 2, 4, 6 and 11 to 15 months. That is, 4 doses. The government has decided that the vaccine schedule will be 2, 4 and 12 months.
This is because when there is an administration of the universal vaccine, that is, when all children are vaccinated, 3 doses seem to be sufficient because the effect of group protection must be added to the individual effect: The more children there are vaccinated, the lower the likelihood of one taking the disease and infecting others. To date, since vaccination was elective, as many children were not vaccinated, the 4 doses were necessary to consider a well vaccinated and protected child.